POSTERIOR NASAL NERVES
Chronic Rhinitis
Affecting millions of patients in the US, chronic rhinitis (CR) is a condition known to adversely impact quality of life and overall productivity.
The condition’s high prevalence and negative impact on sleep, cognitive functioning, and mood, and ultimately on quality of life and performance at school or work, make CR a global public health issue.2

Individuals with rhinitis are 1.5 to 4.5 times more likely to suffer from comorbid conditions including asthma, eczema, migraine, and depression3

Individuals with allergic rhinitis had significantly higher productivity loss compared with other chronic conditions.4

80% of patients with moderate-to-severe rhinitis reported impairment in daily activities5
Currently available treatments have been shown to be effective in interrupting posterior nasal nerves. However, not all patients have adequate resolution of their symptoms. One hypothesis for this lack of improvement is adequate treatment of nerves due to natural anatomic variations that are not addressed by current devices.6
EXPERIENCE THE DIFFERENCE
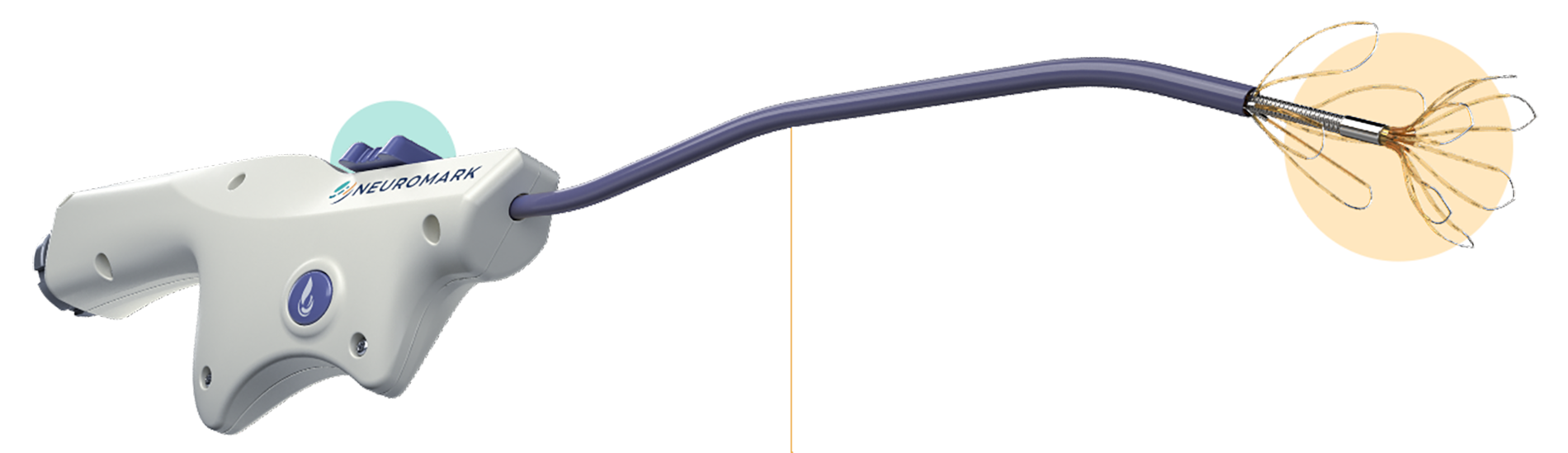
The NEUROMARK® system’s unique device and intelligent technology platform enables Otolaryngologists to treat a broad range of chronic rhinitis patients with CONTROL, CONFIDENCE, and COMFORT.
The NEUROMARK System was designed to treat chronic rhinitis (CR) symptoms with control, confidence, and comfort – and that’s why Dr. Yen is grateful to have it in his clinical toolbelt. See for yourself how your CR patients could benefit from the NEUROMARK procedure.
THE NEUROMARK SYSTEM
Our Technology
NEUROMARK is designed to consistently provide easy access and treatment coverage, including of the posterior regions of the lateral nasal wall.

CONTROL
Achieve precise placement and maximal treatment coverage across posterior nerves and accessory pathways.
More posterior placement1
- Proximal and distal flexible leaflets conform to patient anatomy, maximizing access to nerve-rich areas on the lateral wall of the nasal cavity, and allowing more posterior placement than other available devices1
- Narrow and malleable shaft enables the physician to shape the device to achieve access, even in challenging anatomy
Maximal treatment coverage
- Numerous microablation sites spread over a wide treatment area provides maximal treatment coverage
- Microlesions disrupt posterior nasal nerve pathway while maintaining mucosal and vascular integrity7-9
CONFIDENCE
The NEUROMARK System uses a proprietary algorithm to deliver controlled RF energy
Smart algorithms prevent over- and under-treatment
- Controlled RF energy creates microlesions at the target site while minimizing collateral injury1
- Impedance thresholds are monitored individually for each leaflet, ensuring treatment is delivered adequately to the intended tissue and discontinued at the optimal time1
Data demonstrates high success rate
- 100% procedural technical success10*

COMFORT
NEUROMARK’s customized treatment offers safe and effective relief of chronic rhinitis symptoms while providing a gentle patient experience.
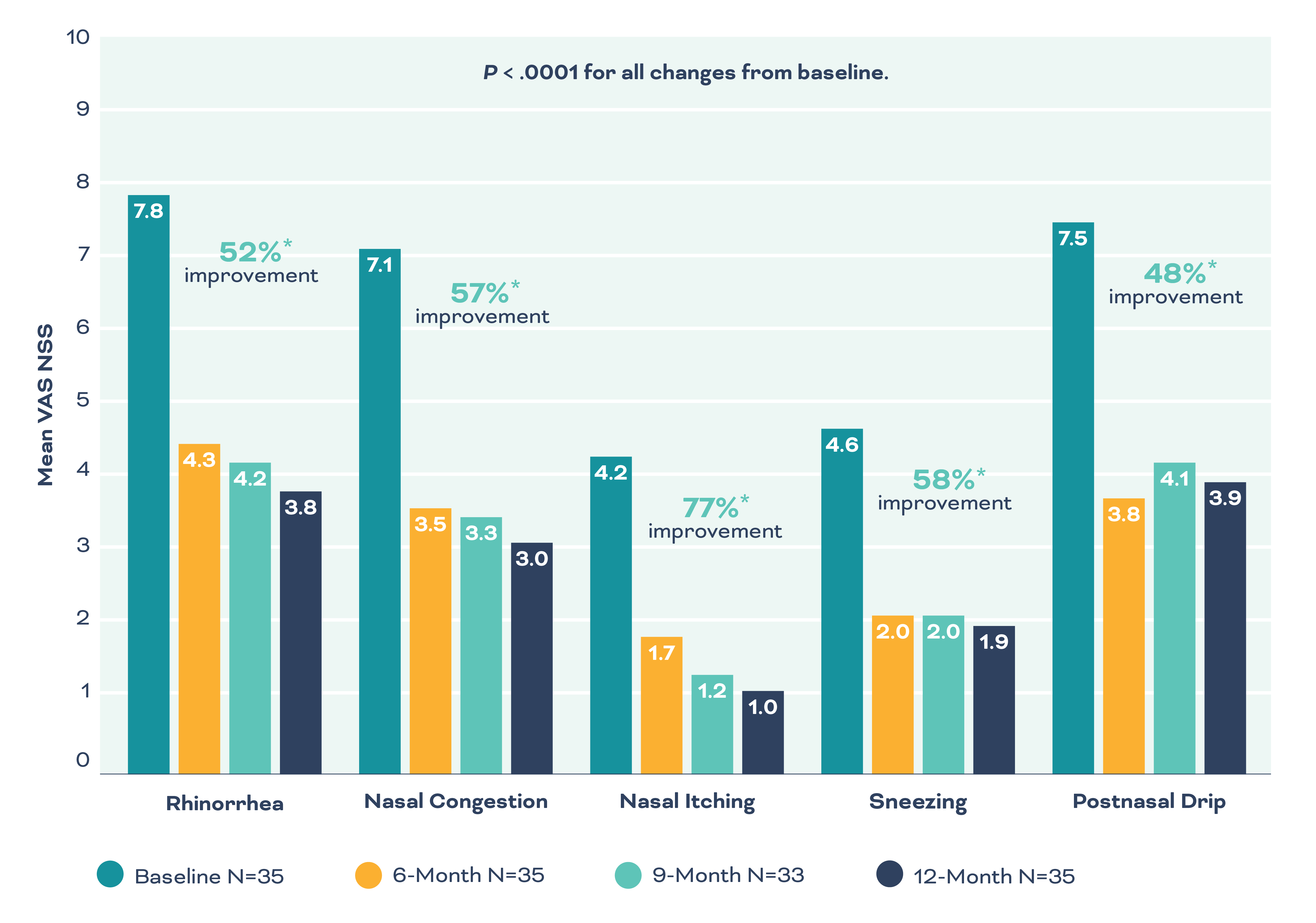
CLARITY Clinical Study1: Statistically significant and clinically meaningful symptom improvements.
Excellent patient tolerability and safety profile demonstrated in 3-month CLARITY study1
- No serious adverse events (SAEs) or post-procedure “ice-cream” headaches reported
- Mean discomfort during procedure was 4.4 (0-10 scale)
- At 24 to 48 hours post procedure, the mean score for discomfort was 1.2, with 72% of participants rating discomfort as 0

WHO IS NEUROMARK RIGHT FOR?





NEUROMARK is an effective option for both long-term and newly diagnosed chronic rhinitis patients.
For patients receiving first-line treatment for chronic rhinitis, NEUROMARK offers results and an optimal patient experience.
Connect with a sales representative to see how NEUROMARK helps you help more patients.

ADD NEUROMARK TO YOUR PRACTICE
Contact Us
*RELIEVE Study based on 1-year exploratory results.
References
- Reh, DD, Lay KL, Davis G, et al. Clinical evaluation of a novel multipoint radiofrequency ablation device to treat chronic rhinitis. Laryngoscope Investig Otolaryngol. 2023. Published online 16 March 2023:DOI: 10.1002/lio2.1040
- Vandenplas O, Vinnikov D, Blanc PD, et al. Impact of Rhinitis on Work Productivi.: A Systematic Review. J Allergy Clin Immunol Pract. 2018;6(4):1274-1286.e9. doi:10.1016/j.jaip.2017.09.002
- Derebery, J., Meltzer, E., Nathan, R. A., et al. (2008). Rhinitis symptoms and comorbidities in the United States: Burden of rhinitis in America survey. Otolaryngology–Head and Neck Surgery, 139(2), 198–205. h.ps://doi.org/10.1016/j.otohns.2008.05.019
- Lamb CE, Ratner PH, Johnson CE, Ambegaonkar AJ, Joshi AV, Day D, et al. Economic impact of workplace productivity losses due to allergic rhinitis compared with select medical conditions in the United States from an employer perspective. Current medical research and opinion 2006;22:1203-10.
- Ozdoganoglu T, Songu M, Inancli HM. Quali. of life in allergic rhinitis. Ther Adv Respir Dis. 2012;6(1):25-39. doi:10.1177/1753465811424425
- Fan T, Chandna M, Gorelik D, et al. Correlation between middle turbinate insertion in relation to sphenopalatine foramen and failure rates of cryotherapy and radiofrequency treatment for chronic rhinitis. Int Forum Allergy Rhinol. 2023;13(1):88-91. doi:10.1002/alr.23058
- TR-018 In vivo assessment of radiofrequency ablation for nasal tissue ablation in an in vivo porcine model-2019
- Haveman et al., 2004. Effects of hyperthermia on the peripheral nervous system: a review. h.ps://www.tandfonline.com/doi/abs/10.1080/02656730310001637631
- Hsu 2014. Significance of Clinical Treatments on Peripheral Nerve and its Effect on Nerve Regeneration. h.ps://pdfs.semanticscholar.org/1cbd/13ec427e4a341413e727820083ca134c2134.pdf
- RELIEVE study on file with Neurent Medical.
- Reh DD, Lay K, Davis G, et al. Long-term outcomes following impedance-controlled radiofrequency ablation for the treatment of chronic rhinitis. Laryngoscope Investig Otolaryngol. 2024;9(3):e1286. Published 2024 Jun 4. doi:10.1002/lio2.1286