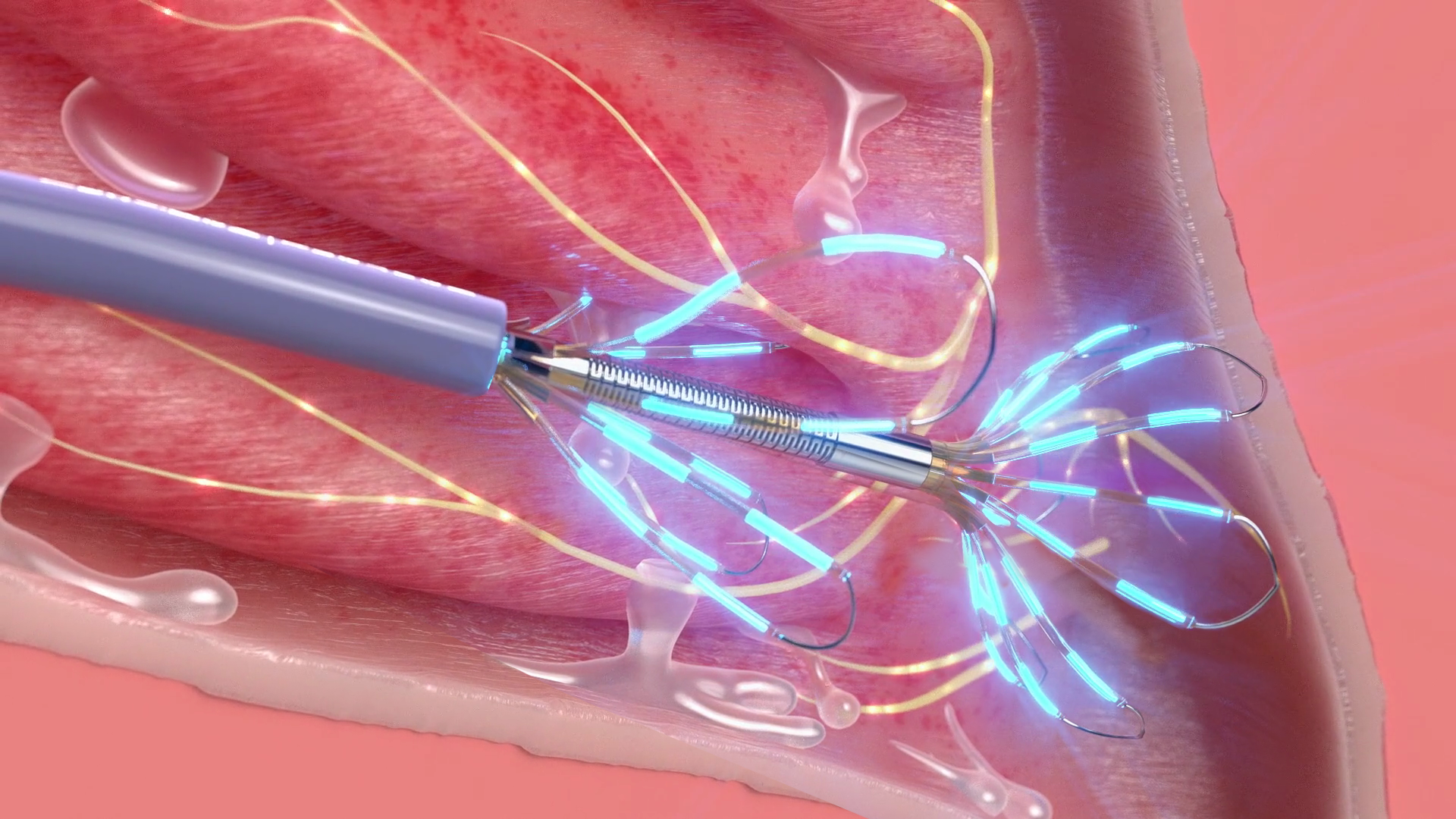
Disclaimer: Illustrations showing electrode activation are indicative only and are not representative of clinical use. During clinical use, the NEUROMARK Console displays the sequence of electrode activation

A peripheral branch of the vidian nerve, the posterior nasal nerve is the dominant source of the parasympathetic, sympathetic, and sensory fibers that innervate the nasal respiratory mucosa.1
Medications used to treat chronic rhinitis (CR), such as intranasal saline, intranasal corticosteroids, oral/topical decongestants, and others, may temporarily relieve symptoms; however, these fail to address the neural source of nasal hypersecretion and many patients do not receive benefit from medical management alone.2
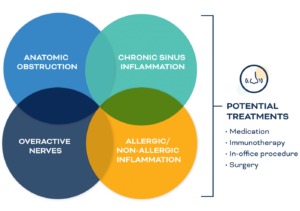
Posterior nasal nerve (PNN) ablation may induce denervation of the nasal mucosa and suppress nasal hypersecretion by blocking autonomic vidian nerve fibers, similar to vidian neurectomy, as well as hypersensitivity by simultaneously blocking sensory nerve fibers to relieve nasal symptoms.1

AAO ENDORSES PNN2
In a 2023 positioning statement on PNN Ablation for the Treatment of Chronic Rhinitis, the American Academy of Otolaryngology — Head and Neck Surgery endorsed the use of PNN ablation for the treatment of medically-refractory chronic rhinitis. The organization noted multicenter, patient-blinded, sham-controlled RCTs have been performed for radiofrequency ablation and cryoablation and have demonstrated:
- Clinical benefit for nasal symptoms
- Statistically significant improvement with treatment, with therapeutic effect
THE EVIDENCE IS CLEAR:
POSTERIOR NERVES MATTER
Recent evidence shows extensive parasympathetic innervation of the nasal cavity, with many fibers posterior to the lateral attachment of middle turbinate (MT).3-5 Clinical data suggest that missing these potential targets with PNN may contribute to low responder rates.3
With traditional approaches using radiofrequency or cryotherapy PNN, randomized controlled trials report that 27%-33% of patients have limited response to treatment.6,7
Non-responders
NEUROMARK is designed to consistently provide easy access and wide treatment coverage including in the posterior regions of the lateral nasal wall
More posterior placement8
- Proximal and distal flexible leaflets conform to patient anatomy, maximizing access to nerve-rich areas on the lateral wall of the nasal cavity, and allowing more posterior placement than some of the other available devices1
- Narrow and malleable shaft enables the physician to shape the device to achieve access, even in challenging anatomy
Maximal treatment coverage
- Numerous microablation sites spread over a wide treatment area provides maximal treatment coverage
- Microlesions disrupt posterior nasal nerve pathway while maintaining mucosal and vascular integrity9-11

Disclaimer: Illustrations showing electrode activation are indicative only and are not representative of clinical use. During clinical use, the NEUROMARK Console displays the sequence of electrode activation
“ELEVATING PNN WITH NEURENT MEDICAL”
Watch the webinar
to see how leading clinicians are utilizing advanced treatment options to disrupt overactive posterior nasal nerves
ADD NEUROMARK TO YOUR PRACTICE

References:
- Nishijima H, Kondo K, Toma-Hirano M, et al. Denervation of nasal mucosa induced by posterior nasal neurectomy suppresses nasal secretion, not hypersensitivity, in an allergic rhinitis rat model. Laboratory Investigation. 2016;96(9):981-993. doi:10.1038/labinvest.2016.72
- Position statement: PNN Ablation for the treatment of chronic rhinitis. American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS). January 17, 2023. Accessed May 21, 2024. https://www.entnet.org/resource/position-statement-posterior-nasal-nerve/.
- Fan T, Chandna M, Gorelik D, et al. Correlation between middle turbinate insertion in relation to sphenopalatine foramen and failure rates of cryotherapy and radiofrequency treatment for chronic rhinitis. Int Forum Allergy Rhinol. 2023;13(1):88-91. doi:10.1002/alr.23058
- Bleier BS, Schlosser RJ. Endoscopic anatomy of the postganglionic pterygopalatine innervation of the posterolateral nasal mucosa. Int Forum Allergy Rhinol. 2011;1(2):113-117. doi:10.1002/alr.20011
- Ogi K, Valentine R, Suzuki M, Fujieda S, Psaltis AJ, Wormald PJ. The anatomy of the foramina and efferent nerve fibers from the pterygopalatine ganglion in posterolateral nasal wall. Laryngoscope Investig Otolaryngol. 2022;7(3):679-683. Published 2022 May 20. doi:10.1002/lio2.808
- Stolovitzky JP, Ow RA, Silvers SL, Bikhazi NB, Johnson CD, Takashima M. Effect of Radiofrequency Neurolysis on the Symptoms of Chronic Rhinitis: A Randomized Controlled Trial. OTO Open. 2021;5(3):2473974X211041124. Published 2021 Sep 10. doi:10.1177/2473974X211041124
- Del Signore AG, Greene JB, Russell JL, Yen DM, O’Malley EM, Schlosser RJ. Cryotherapy for treatment of chronic rhinitis: 3-month outcomes of a randomized, sham-controlled trial. Int Forum Allergy Rhinol. 2022;12(1):51-61. doi:10.1002/alr.22868
- Reh DD, Lay K, Davis G, et al. Clinical evaluation of a novel multipoint radiofrequency ablation device to treat chronic rhinitis. Laryngoscope Investig Otolaryngol. 2023;8(2):367-372. Published 2023 Mar 16. doi:10.1002lio2.1040
- TR-018 In vivo assessment of radiofrequency ablation for nasal tissue ablation in an in vivo porcine model-2019
- Haveman J, Van Der Zee J, Wondergem J, Hoogeveen JF, Hulshof MC. Effects of hyperthermia on the peripheral nervous system: a review. Int J Hyperthermia. 2004;20(4):371-391. doi:10.1080/02656730310001637631
- Hsu M. Significance of clinical treatments on peripheral nerve and its effect on nerve regeneration. Journal of Neurological Disorders. 2014;02(04). doi:10.4172/2329-6895.1000168
Terms & Conditions | Privacy Statement | Cookie Settings | Website Accessibility
The materials on this website are for your general educational information only. Information you read on this website cannot replace the relationship that you have with your healthcare professional. We do not practice medicine or provide medical services or advice as a part of this website. You should always talk to your healthcare professional for diagnosis and treatment.
NEUROMARK® device may be used in patients with recurring stuffy and/or runny nose. Your physician will need to determine if your condition is one that may benefit from NEUROMARK. Possible side effects include but are not limited to post-operative bleeding, pain or discomfort, allergic reaction to anesthesia or other medication administered during the procedure, and infection. Your condition may not respond to the treatment. To learn more about this procedure and potential risks, ask your physician.
INDICATIONS FOR USE: The NEUROMARK® System is indicated for use in otorhinolaryngology (ENT) surgery for creation of radiofrequency (RF) lesions to disrupt posterior nasal nerves in patients with chronic rhinitis.
Please see Instructions for Use (IFU) for a complete listing of warnings, precautions, and adverse events.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
NEUROMARK is trademark of Neurent Medical. PAM072r01 July 2023 Copyright © 2024 Neurent Medical, Inc.